The compound(s) having peroxide linkage is(are) (A) H 2 S 2 O 7 𝐻 2 𝑆 2 𝑂 7 (B) H 2 S 2 O 8 𝐻 2 𝑆 2 𝑂 8 (C) H 2 S 2 O 5 𝐻 2 𝑆 2 𝑂 5 (D) H 2 S O 5
Understanding Peroxide Linkage
A peroxide linkage refers to an oxygen-oxygen single bond (O-O), commonly found in peroxides (e.g., hydrogen peroxide, H₂O₂). To identify which sulfur-oxygen compounds contain this linkage, we analyze their structures.
Analyzing Each Option
(A) H₂S₂O₇ (Pyrosulfuric Acid)
Structure:
Bonds:
Contains S-O-S (sulfate bridge) but no O-O bond.
Conclusion: ❌ No peroxide linkage.
(B) H₂S₂O₈ (Peroxydisulfuric Acid)
Structure:
Bonds:
Contains a peroxide linkage (O-O) between the two sulfate groups.
Conclusion: ✅ Has a peroxide linkage.
(C) H₂S₂O₅ (Disulfurous Acid)
Structure:
Bonds:
Contains S-S and S=O bonds but no O-O bond.
Conclusion: ❌ No peroxide linkage.
(D) H₂SO₅ (Peroxymonosulfuric Acid, "Caro's Acid")
Structure:
Bonds:
Contains a peroxide linkage (O-O) at the terminal -OOH group.
Conclusion: ✅ Has a peroxide linkage.
Final Answer
The compounds with peroxide linkages are:
(B) H₂S₂O₈ and (D) H₂SO₅.
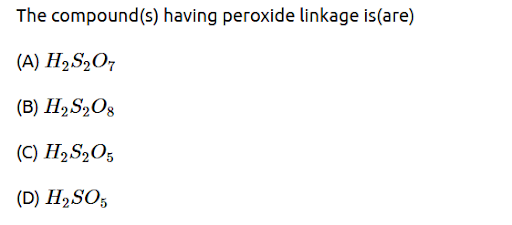
Comments
Post a Comment